Spatial Distribution of Lead, Zinc,
Cadmium, and Nickel in the South Creek Watershed, SW Missouri
Jimmy Trimble
GLG 581
Final Project
05-01-01
ABSTRACT
Many non-point source contaminants
in fluvial systems originate in urban landscapes. Metals are an important component of urban run off and are
attributed to water quality degradation.
Detention ponds can act as environmental buffers and impede the
downstream transport of these contaminants.
The current study examines metal contaminants in the South Creek
Watershed which drains the urban area Springfield, Missouri. South Creek is heavily affected by urban
landuse, however, an in stream detention pond exists on the creek and may serve
to trap sediment bound metals. To test
the effectiveness of the detention pond, sediment samples were collected from
seven sites upstream of the pond and seven sites downstream. The <0.63 mm size fraction of each sample was analyzed for lead, zinc,
cadmium, and nickel using an Inductively Coupled Plasma (ICP)
spectrometer. Metal concentrations for
lead, zinc, and cadmium generally decrease downstream. Nickel concentrations are more variable
throughout the watershed. A T-test
indicates that concentrations of zinc and cadmium are significantly different
upstream and downstream of the pond at the 90% confidence level. Lead and nickel concentrations are not
significantly different upstream and downstream of the pond at the 90%
confidence level. The detention pond in
the South Creek Watershed is not a significant trap for sediment bound metals
originating in Springfield, MO.
INTRODUCTION
Recent water quality degradation in streams
has been partly attributed to the affects of urbanizing areas. Degradation has been linked both to elevated
nutrient input and metal input. Both
point and non-point sources are to blame for water quality degradation. Much focus has been placed on regulating
point sources such as industrial discharge and wastewater treatment
plants. More and more research is
focusing on the affects of urban non-point sources such as parking lots, lawn
care, construction sites, and roads.
A related body of research has
developed that focuses on the usefulness of detention ponds and constructed
wetlands as environmental buffers against these non-point source
contaminants. These areas serve to trap
sediment and the associated pollutants including metals and nutrients. Researchers have investigated the usefulness
of wetlands and detention ponds to attenuate the impact of urbanizing areas and
contaminant introduction in general.
Studies have also examined phytoremediation in constructed wetlands as
opposed to terrestrial phytoremediation.
When compared to terrestrial phytoremediation, the anoxic soils in
wetland areas immobilize pollutants and metals are held in nontoxic forms
(Horne, 2000).
Watersheds that drain the urbanizing
area of Springfield, Missouri suffer from degraded water quality due to
non-point source introduction of metals and nutrients. The current study is focused primarily on
four metal contaminants. The metals examined
in this study are lead (Pb), zinc (Zn), Cadmium (Cd), and Nickel (Ni). The streams that drain this area empty into
tablerock lake, a very tourist dependent area.
Degraded water quality has a detrimental affect on this tourism.
There is a lack of research
concerning urban area influence on metal contaminants in Ozark streams. Much research has been conducted on the
affects landfills have on sediment bound metal concentrations in Ozark streams
(Mantei and Coonrod, 1989; Mantei and Sappington, 1994). Another study has evaluated the phases of
metal contamination from several sources, including a relatively densely
populated area of Springfield, MO. (Mantei and Foster, 1991). However, this study did not evaluate metals
solely originating in the urban area from non-point sources.
Another study has examined the
importance of various urban functions as sources of metals (Xanthopolous and
Hahn, 1993). Xanthopolous and Hahn
(1993) concluded that urban streets are an important non-point source of
metals. A study by the U.S.
Environmental Protection Agency (EPA) (1990) identified sources of lead and
zinc in an urban setting. According to
this study, sources of lead include: gasoline; batteries; paint; and lead
pipe. Sources of zinc include: metal
corrosion; tires; road salt; wood preservatives; and paint. Bannerman (1991) related increases in zinc
and lead concentrations to increases in traffic densities. Marsalek (1986) studied urban runoff in both
water column samples and sediment samples and concluded that the most prominent
toxic contaminants in urban runoff are trace metals. The most frequently encountered metals were zinc (98% of samples),
nickel (87% of samples), and lead (78% of samples). Another study by Pitt and Baron (1989) studied important sources
of dissolved metals in the urban system.
Table 1 illustrates their findings for lead, zinc, cadmium, and
nickel. The authors found that roof
runoff has the highest input of dissolved zinc. According to the authors parking areas are important sources of
dissolved nickel and lead. Streets and
vehicle service areas are important sources of cadmium.
Table 1 Dissolved
Metal Concentrations from Urban Runoff (adapted from Pitt and Baron,1991)
|
Constituent mg/l |
Urban Sources |
|||||
|
|
roofs |
parking |
storage |
streets |
vehicle service |
landscapes areas |
|
Cadmium |
0.8-30 |
0.7-70 |
2.4-10 |
0.7-220 |
8-30 |
0.04-1 |
|
Lead |
13-170 |
30-130 |
30-330 |
30-150 |
75-110 |
9.4-70 |
|
Nickel |
5-70 |
40-130 |
30-90 |
3-70 |
35-70 |
30-130 |
|
Zinc |
100-1580 |
30-150 |
66-290 |
58-130 |
67-130 |
32-1160 |
Specifically, this study is focused
on sediment bound metal contamination in South Creek, a tributary of Wilson
Creek and ultimately the James River.
South Creek is strictly an urban stream and suffers from the associated
land uses. South Creek heads near the
Battlefield Mall and essentially runs due west to its confluence with Wilson’s
Creek (Figure 1). A large portion of
South Creek is channelized and is confined to a concrete channel. South Creek receives many small tributaries
that run through various subdivisions and other urban landscapes. A detention pond exists in the course of
South Creek upstream of its confluence with Wilson’s Creek. This study is focused on assessing sediment
bound metal levels upstream and downstream of this detention pond. The research hypothesis is that this pond
will attenuate the downstream affects of urbanization. Metal concentrations in stream sediments
should be much lower than concentrations above the pond and concentrations of
metals in the pond sediment should be the most elevated.
![]()
![]()
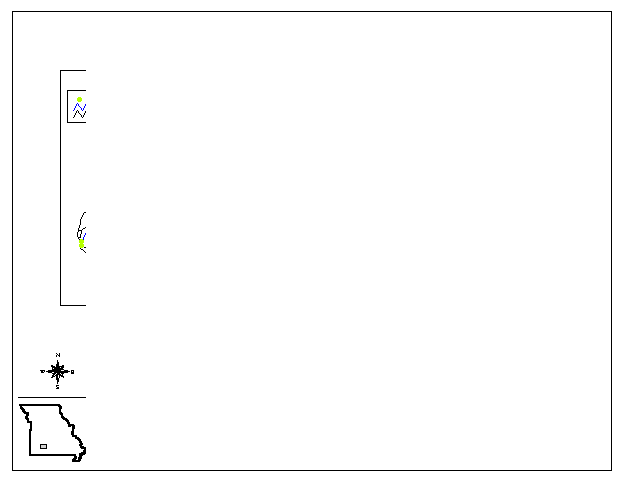
Figure
1 Regional Setting and Sample Site
Locations
METHODS
Methodology for the current study
consisted of field work, sample preparation and analysis, data analysis, and gis
analysis.
Field
Methods Field methods consisted largely
of collecting sediment samples from both in-channel and pond sites. Sediment samples were collected from
fourteen in-channel sites (Figure 1).
Fine grained sediment was collected from seven sites upstream of the
pond and seven sites were sampled downstream of the pond. The samples were
collected at an approximately equal interval both downstream and upstream of
lake. Seven samples were also taken from
a pit dug into the pond sediment. The pit
was dug to a sufficient depth to represent the sediment in the pond. The
geochemical results of these seven samples were averaged to represent the level
of contamination in the lake sediment.
Each sample
site was recorded with a handheld GPS unit for entry into a GIS. After sample
collection, each was immediately bagged, sealed, and labeled for transport back
to the lab.
Sample
Preparation and Analysis Upon
delivery back to the geomorphology lab at Missouri State University,
each sample was wet sieved to pass through a 0.63 mm mesh sieve. The <0.63 mm fraction of the samples were retained for geochemical
analysis. When necessary, samples were
disaggregated with a mortar and pestle to pass through the brass sieve. The sieved sediment was poured into a 250 ml
beaker. Two grams of the sample were
weighed and retained in a sample vile for analysis. An acid solution was created to dissolve samples and suspend the
metals. Dionized water was used to
dilute nitric acid (HNO3 ) to a 500 ml, 2N solution. The 2g portion of each sample was treated
with a single wash of the 2N HNO3 solution and placed in a shaker
bath at 80°C for 24
hours. Once the samples were thoroughly
dissolved they were centrifuged in order to further separate the dissolved
sample from any particulate matter that remained. The dissolved sample solution for each sample was decanted into a
vile for geochemical analysis. An
Inductively Coupled Plasma (ICP) spectrometer was used to analyze the metal
concentrations in each sample.
Prior
to geochemical analysis, two standards and one blank were created in order to
correctly calibrate the ICP for analysis of Pb, Zn, Cd, and Ni. A two point standard method was used.
Standards were mixed using metal stock solution, nitric acid, and diionized
water. One standard was created at the
estimated low concentration of the samples which was estimated to be 20
ppm. The standard was diluted by a
factor of ten, so the actual concentration evaluated by the ICP was 2 ppm. Another standard was created at 100 ppm and
was likewise diluted by a factor of 10 to 10 ppm. A “blank” was also created to be analyzed by the ICP. The blank consisted of only diionized water
and nitric acid. The blank was analyzed
by the ICP in order to calibrate the instrument for any metals that may be
present in these substances.
The
ICP instrument was programmed to analyze the wavelength emitted from the four
metals under investigation. Initially
the two standards and the blank were analyzed in order to properly calibrate
the machine. Each sample was
subsequently analyzed and a printout of the results was produced. After analysis the samples were discarded
and the lab equipment cleaned.
Data
Analysis The geochemical results were
recorded in a spreadsheet program for analysis and display. Microsoft Excel was used to produce trend
charts of the spatial distribution of the four metals under examination. The geochemical results were transferred to
another software package and a student t-test was conducted in order to
compare the mean of the samples upstream of the pond with the mean of the
samples collected downstream of the pond.
GIS
Methodolgy A GIS was used to
organized and display spatial data.
Relevant spatial data layers were downloaded from ESRI Inc. (www.esri.com) and stored
in the GIS. Urban areas, street files,
and stream files were downloaded and visually displayed. Sample site locations were downloaded from
the GPS unit to a PC and combined with the other data layers. The sample site theme was joined with the
spreadsheet of the sample analysis in order to qualitatively assess and display
downstream spatial distribution of
zinc, lead, cadmium, and nickel within South Creek.
Results
The original
research hypothesis is that the control sample sites (upstream of pond) should
have higher concentrations than sample sites downstream of the pond. The concentrations of Pb, Zn, Ni, and Ca at
the all sample sites are shown in Table 2.
The mean concentrations for the four metals at both upstream and
downstream sites are given in Table 3.
Average metal concentrations in the pond (site 8) are not abnormally
high and do not suggest that the pond is a major sink for metals.
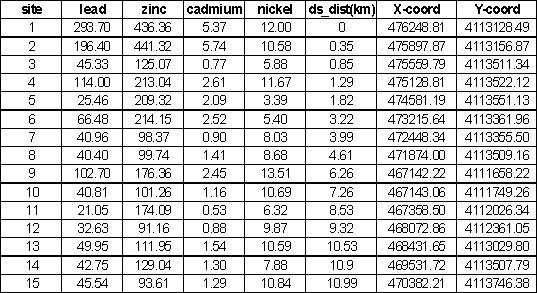
Table 2. Metal Concentrations, upstream of pond
(1-7), in pond (8) and downstream of pond (9-15).
Table 3. Mean Metal Concentrations Upstream and Downstream of Pond
|
Metal |
Upstream
Mean (ppm) |
Downstream Mean (ppm) |
|
Lead |
111.76 |
47.92 |
|
Zinc |
248.23 |
125.35 |
|
Cadmium |
2.856 |
1.308 |
|
Nickel |
8.135 |
8.409 |
Concentrations
for all of the metals except Ni generally decrease longitudinally downstream (Figures
2-5). All metals have a second major
spike in concentrations downstream of the pond and then generally decrease
longitudinally. Nickel has a rather sporadic downstream trend, but also spikes
downstream of the pond (Figure 5).
Nickel is a component in some fertilizers and its trend could be
influenced by the non-point sources of fertilizer throughout the stream. At the location where Ni spikes downstream
of the pond is a golfcourse that could be contributing to the high Ni
concentrations due to fertilizing of grass.
Cadmium, zinc, and lead have similar downstream trends reflecting the
similarity in sources of these metals (Figures 2,3,4).
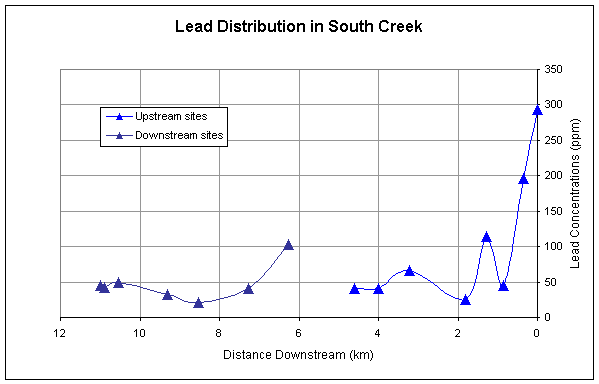
Figure 2.
Downstream distribution of lead sampled at sites upstream and downstream
of pond.
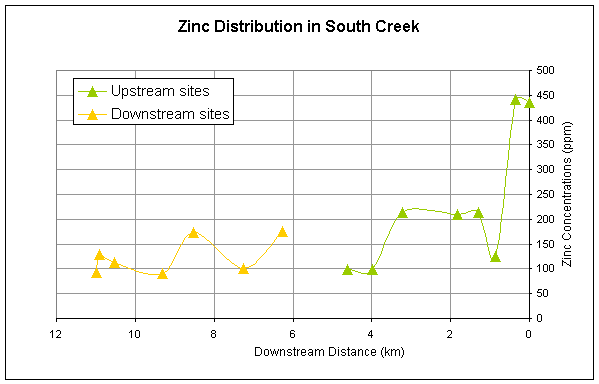
Figure 3.
Downstream distribution of zinc sampled at sites upstream and downstream
of pond.
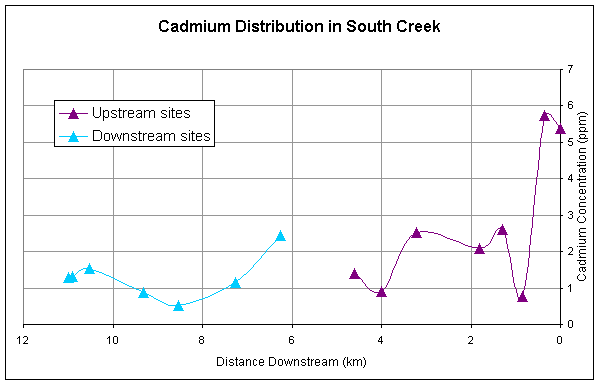
Figure 4. Downstream
distribution of cadmium sampled at sites upstream and downstream of pond.
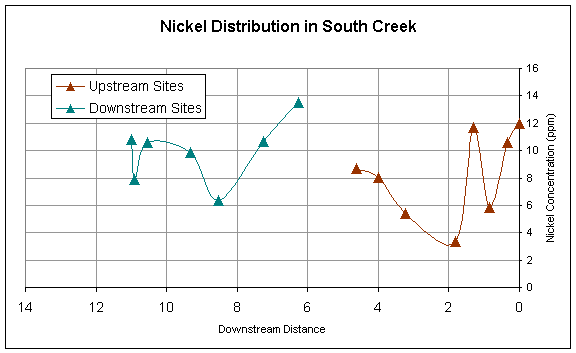
Figure 5.
Downstream distribution of Nickel sampled at sites upstream and
downstream of pond.
To test the
research hypothesis a two sample t-test was conducted to evaluate the
differences in the mean concentrations of the metals upstream and downstream of
the pond. Table 4 displays the t-values and two tailed critical values
for each metal. Supporting the research
hypothesis would require the samples downstream to be significantly lower than
samples upstream of the pond. Lead
concentrations were not significantly different upstream and downstream of the
pond at the 0.1 alpha (90%) confidence level.
Zinc concentrations are significantly different upstream and downstream
at the 0.1 alpha (90%) level. Cadmium
concentrations were also significantly different at the 0.1 alpha (90%)
confidence level in the upstream sites vs. the downstream sites. Mean nickel concentrations were not
significantly different at the 0.1 alpha level (90%). The results for zinc and cadmium support the research hypothesis
while the results for lead and nickel reject the hypothesis.
Table 4. t-test results for upstream and
downstream samples
|
Metal |
Upstream Mean |
Downstream Mean |
0.1 Alpha |
|
|
|
|
|
t-value |
critical value |
|
Zinc |
248.23 |
125.35 |
2.2786 |
1.8945 |
|
Lead |
111.76 |
47.92 |
1.645 |
1.895 |
|
Cadmium |
2.856 |
1.308 |
1.976 |
1.895 |
|
Nickel |
8.135 |
8.409 |
0.131 |
1.796 |
CONCLUSION
Two
of the metals, Zn and Cd, examined in the study are slightly affected by the
pond. Two of the metals, Pb and Ni, are
not affected by the pond. The most likely
reason why the pond does not greatly reduce downstream metal concentrations is
the fact that many non-point sources of metals exist downstream of the
pond. Any attenuation of metal
concentrations by the pond is negated by sources downstream. In fact, the sediment contained in the pond
does not have an abnormally high metal content.
Another
complication with sediment bound metal transport in South Creek is sediment
residence times. Most sediment does not
reside in the channel for long periods of time, thus reducing the absorption
time of metals by the sediment. South
Creek is very flashy in nature and much of the channel is concrete. This scenario creates an efficient conduit
for material carried by the stream. Any
sediment deposited in the channel is most likely completely washed from the
system during the next flow event. It
could be suggested, by looking at the trend charts, that metal enrichment in
South Creek is nearly syngenetic in nature.
The metals get deposited with the sediment and are washed out of system
during the next event. The sediment
does not remain in the channel where it has a chance to absorb metals from
subsequent flow events.
Future
recommendations would be to sample more extensively and to sample Wilson’s
Creek. Samples taken in Wilson’s Creek
upstream and downstream of the confluence with South Creek would reveal metal
inputs from South Creek. This type of
sampling scheme would provide more evidence for amounts of metals that may be
originating from the urban landscape.
BIBLIOGRAPHY
Bannerman,
R. ,1991, “Pollutants in Wisconsin Stormwater”, Unpublished report by the
Wisconsin Department of Natural Resources, Madison, WI.
Horne, J.H.,
2000, “Phytoremediation by Constructed Wetlands”. In: Phytoremediation of Contaminated Soil and Water.
(Terry, N. and Banuelos, G. eds.) pp13-39, Lewis Publishers, Boca Raton,
London, New York, Washington, D.C.
Mantei, E.J.
and Coonrod, D.L., 1989, “Heavy Metal Content in the Stream Sediments
Adjacent to a Sanitary Landfill.” Environmental
Geology and Water Science, v.13
no. 1, pp.51-58.
Mantei, E.J.
and Foster, M.V., 1991, “Heavy Metals in Stream Sediments: Effects of
Human Activities.” Environmental Geology and Water Science,
v.18
no. 2, pp.95-104.
Mantei, E.J.
and Sappington, E.J., 1994, “Heavy metal concentrations in sediments of
Streams affected by a sanitary
landfill: A comparison of metal enrichment in two size sediment fractions.” Environmental
Geology, v.24, pp.287-292.
Marsalek,
J., 1986, “Toxic contaminants in urban runoff” In: Urban Runoff
Pollution
(Torno, H., Marsalek, J., and
Desbordes, M., eds.) pp 39-57, Springer
Verlag, Berlin.
Pitt, R. and
Barron, P., 1989, “Assessment of urban and industrial stormwater runoff
toxicity and the
evaluation/development of treatment for runoff toxicity abatement-Phase
I.” A report for the U.S. Environmental
Protection Agency, Storm and Combined Sewer Pollution Program, Edison, NJ.
U.S. EPA,
1990, National Water Quality Inventory – 1988 Report to Congress. EPA 440-4-90-003,
Office of Water, Washington DC.
Xanthopolous,
C. and Hahn, H.H., 1993, “Anthropogenic wash-off from street surfaces” In:
Proceedings of the Vith International Conference on Urban Storm Drainage,
Niagara Falls, Ontario, Canada. pp. 417-422. (Marsalek,J. and Torno, H.C.
eds.). Seapoint Publishing, British Columbia.