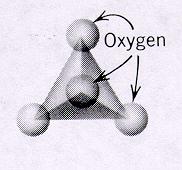
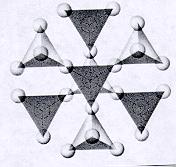
| Nesosilicates units of independent tetrahedra p:q = 1:4 or (3:12)--basic unit is (SiO4)-4 or (Si3O12)-12 4 oxygens on each unit are free to connect with cations of other polyhedra and in turn more independent tetrahedra units can connect with these polyhedra because the SiO4 tetrahedra are independent, the crystal habit of minerals is equidimensional and pronounced cleavage is absent |
 |
| Sorosilicates units of two tetrahedra sharing one common oxygen p:q = 2:7--basic unit is (Si2O7)-6 6 oxygens on each unit are free to connect with cations of other polyhedra and in turn more of these soro- units can attach to these polyhedra |
 |
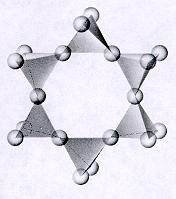
| Cyclosilicates closed ring units of tetrahedra each sharing 2 oxygens p:q = 1:3 (6:18)--basic unit is (Si6O18)-12 12 oxygens on each unit are free to connect with cations of other polyhedra and in turn more of these ringed units can attached to these polyhedra atoms and ions can be trapped within the open spaces of the rings |
 |
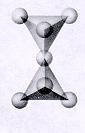
| Inosilicates continuous single chain units of tetrahedra each sharing 2 oxygens p:q = 1:3 (1:3 or 2:6)--basic unit is (SiO3)-2 or (Si2O6)-4 single chained units are connected by other polyhedra units--cleavage along the connected polyhedra forms the characteristic 90 degree 2 directional cleavage typical in this subclass |
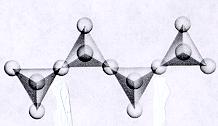 |
| Inosilicates continuous double chain units of tetrahedra each sharing 2 and 3 oxygens alternately p:q = 4:11 (4:11 or 8:22)--basic unit is (Si4O11)-6 or (Si8O22)-12 double chain units are separated by connected polyhedra units--cleavage along connected polyhedra forms the characteristic 120--60 degree cleavage typical in this subclass ions and atoms can be trapped between open spaces between tetrahedra |
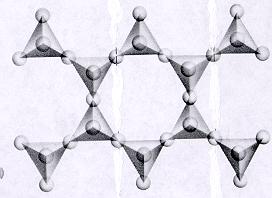 |
| Phyllosilicates continuous sheet units of tetrahedra each sharing 3 oxygens p:q = 2:5 (2:5 or 4:10)--basic unit (Si2O5)-2 or (Al Si3O10)-5 where Al substitutes for Si -sheet units are separated by connected polyhedra units--cleavage along connected polyhedra forms the characteristic perfect cleavage in one direction (sheet cleavage) typical in this subclass atoms and ions can be trapped in open spaces between tetrahedra units |
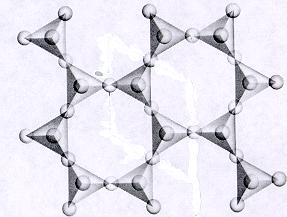 |
| Tectosilicates continuous framework of tetrahedra each sharing all 4 oxygen atoms--large substitutions of Al+3 for Si+4 in the tetrahedron allows polyhedra to connect to oxygens in the tetrahedra with both Al and Si bonds since the sum of e.v.of Al and Si will not equal the valence of oxygen p:q = 1:2, 2:4, 4:8, 6:12--basic units can be (SiO2), (AlSiO4)-1, (Al2Si2O8)-2, or (Al2Si4O12)-2 |
 |
|
|
| D. Some Mineral Groups and Series
in the Silicate Subclasses -part of the material you will be tested on in lab # 5 will be the identification by name of silicate minerals underlined below using hand specimens--information given below for the minerals and additional information for the same treated in the Mineral Classification and Physical Properties sections will be material subject for examination in lecture exam #4 1. Nesosilicates olivine solid solution series--important in igneous rock forming processes garnet isomorphic group--often occur as dodecahedron crystals- pyrope, almandine, grossularite--abundantly found in metamorphic rocks zircon can form metamict structure kyanite belongs to a polymorphic group (Al2OSiO4) topaz staurolite can form cross twinning 2. Sorosilicates hemimorphite-- often found as bladed crystals epidote forms an isomorphic group--important rock forming mineral allanite can form metamict structure--characteristally black with no cleavage 3. Cyclosilicates ( hardness is high, comprises many gemstone varieties and poor cleavage is abundant) beryl gemstone varieties include: emerald (deep green and transparent); aquamarine (pale- greenish-blue transparent); morganite (rose transparent) cordierite often displays dichroism tourmaline (commonly shorl or the black variety) gemstone varieties include: rubellite (red-pink); indicolite (dark blue)
4. Inosilicates (includes the pyroxene
group--single chain minerals lacking (OH)x and amphibole
5. Phyllosilicates (includes the
clay and mica minerals-perfect 1 directional cleavage with 6. Tectosilicates ( framework
structure in which there is great amounts of Al-Si substitution) |
|