Introduction
Location
Geographic setting
Figure 1: Overview of study area
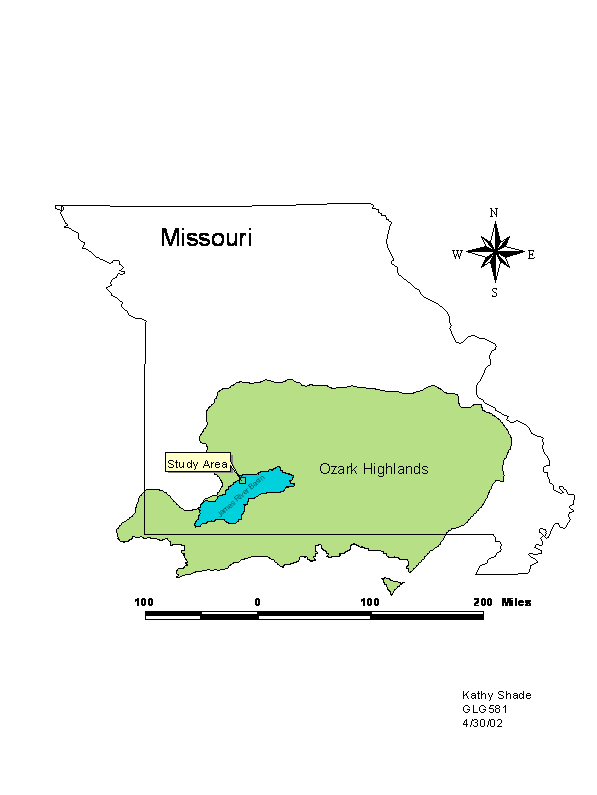
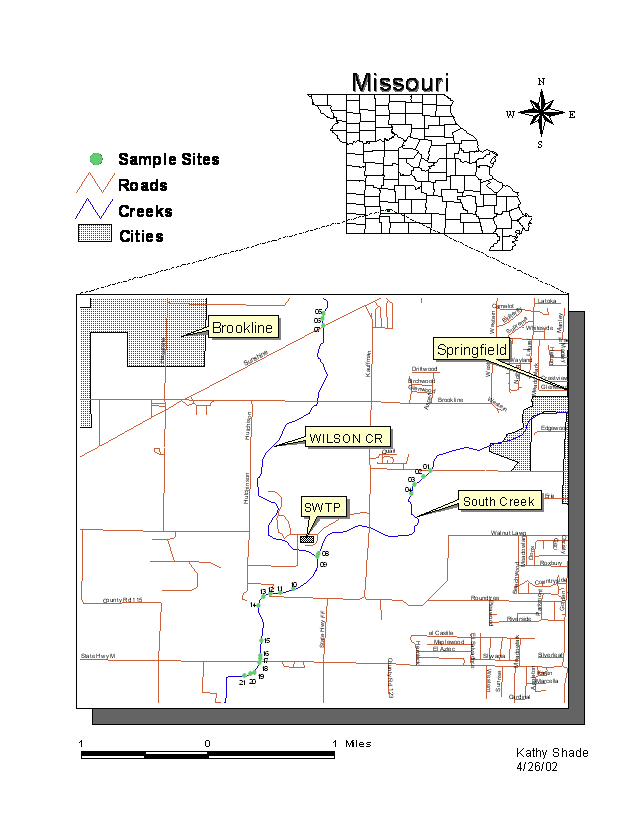
Figure 2: Detail of
Geologic setting
The area is dominated by limestone, shale and
sandstone. Most of the bedrock surface in the
Groundwater may flow relatively freely through and between four limestone formations before encountering the Northview formation, a shale or siltstone layer that acts as an aquitard and restricts flow and dips slightly from northeast to southwest. The Northview formation generally hinders downward flow of groundwater except where it is breached by wells or faults or fracture zones.
Below the
Northview formation is the Ozark aquifer – the primary source for municipal and
industrial wells in the
Land use
Land use in the basin is primarily
agricultural (mostly pasture and some row cropping), some forested, and
increased urban development (Figure 3). The only major city in the basin is
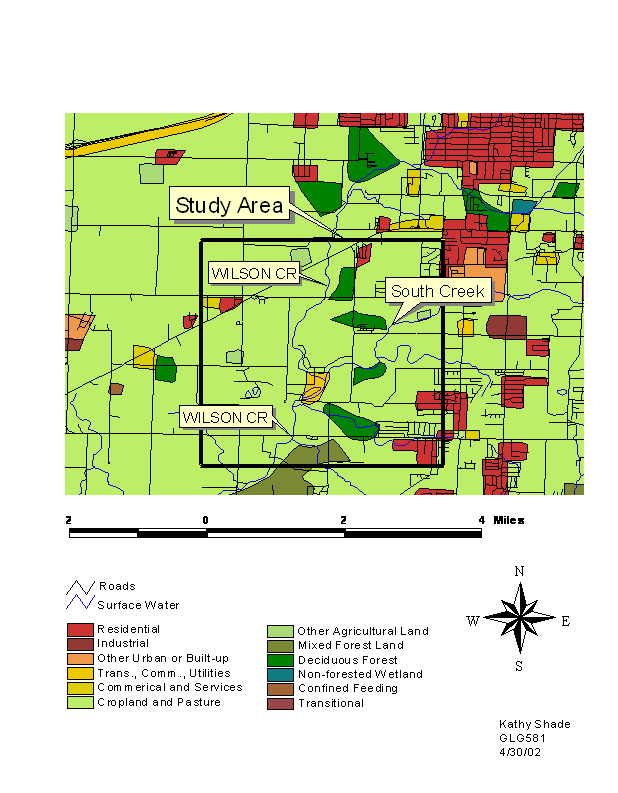
Figure 3: Land use around study area
Hydrology
In the
Streams in the
Both
In addition to the above-mentioned chlordane and sewage problems, the state has identified other surface water concerns within the basin. These include wastewater discharges from other point and non-point sources, agricultural runoff, spills or leaks of materials, landfills, biocides, storm water runoff/flooding, solid wastes, destruction or deterioration of riparian zones, and high volume of water use.
History of emission sources
Within the
Point sources
The Southwest
Wastewater Treatment Plant (SWTP) is located at the confluence of
Biosolids removed from the wastewater are disposed of in two ways: sludge thickening and dispersal as fertilizer on fields and/or dewatering and mixing with an amendment such as woodchips or sawdust then composting for 25 days. Compost can be stored until is can be used on fields.
Water is tested continuously (approximately 5000 samples per month) to ensure that the plant meets all Federal and State standards. Typical effluent water quality from the STWP is summarized below in Table 1:
Table 1: Typical effluent water quality from the SWTP
|
Waste
Component |
Concentration |
|
Biochemical Oxygen Demand |
< 2 mg/L |
|
Total Suspended Solids |
< 2 mg/L |
|
Ammonia Nitrogen |
< 0.1 mg/L |
|
Dissolved Oxygen |
> 20 mg/L |
|
Fecal Coliform |
< 10 colonies per 100 mL |
|
Passes Whole Effluent Toxicity Test |
|
|
pH |
7.10 std. Units |
|
Copper |
15 ug/L |
|
Chromium |
< 10 ug/L |
|
Zinc |
40 ug/L |
|
Cadmium |
< 5 ug/L |
|
Lead |
< 20 ug/L |
|
Nickel |
< 10 ug/L |
|
Mercury |
< 0.2 ug/L |
|
Silver |
< 5 ug/L |
|
Arsenic |
< 20 ug/L |
|
Cyanide |
< 10 ug/L |
|
Total Toxic Organics |
Below detection limits |
City of
In the past,
copper has been the most limiting factor for
Other
point sources within the basin are residential areas including subdivisions
such as
Non-point sources
Non-point sources
in the basin could contribute as much as 70% of the nutrient pollution within
the basin (City Utilities 2001). Runoff from dairy cattle operations, poultry
and turkey farms, sedimentation, sludge application, coal pile runoff, volatile
organics, and seepage from septic systems are all probable contributors. Storm water overflow continues to be a
problem for the city of
Hypothesis
If the Southwest
Wastewater Treatment Plant were a significant source of heavy metal and
nutrient contamination in
Methods
Choice of sediment size and collection of samples
According to a study by Mantei, Ernst and Zhou (1993), sediment size and sieving technique affect the concentration of metals that are adsorbed to and can be extracted from the sediments. They found that “homogeneity of metals in the very fine sand size sediments appears to be relatively high” and that the “sediment size used for chemical analysis should be obtained through sieving at the collection site.” They further cautioned that the sediments should not be crushed or sieved in the laboratory because this could result in false metal quantities for the elements. The collection and preparation techniques used in this study followed the recommendations given in the Mantei, Ernst, and Zhou paper.
Twenty-one samples
were collected from the two study streams.
Four South Creek sediment samples were collected as control samples
upstream from the SWTP along the South Creek Greenway south of
Lab preparation of samples
The sediments were transferred to 150 mL glass beakers and rinsed with double-deionized water until all visible organic matter and suspended particles were removed. The samples were then dried in an oven for 24 hours. Each sample was gently stirred with a glass rod until all visible clumps were disaggregated.
Each sample was massed on an electronic balance and a 1.000-gram portion was removed for testing. The sample was placed in a clean plastic centrifuge tube and 20mL of 3M HNO3 was added. Sample bottles and contents were placed in a water bath and agitated for 24 hours. The samples were centrifuged and the supernatant decanted into clean plastic sample bottles.
ICP setup and chemical analysis
Three standard solutions for each of the metals (2, 5, and 10 ppm), phosphorus (20, 50, and 100 ppm) and a blank sample of HNO3 for ICP analysis were prepared.
An ICP spectrophotometer was programmed and calibrated using the previously prepared standards for each of the elements to be analyzed. The concentrations of each element in each of the test and control samples were determined by standard ICP procedures. A printout of the results was produced for analysis.
Tables and trend charts
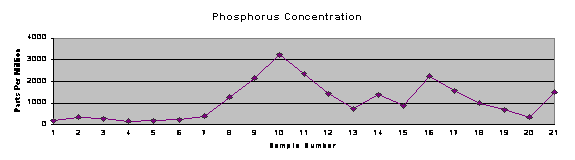
Results of the ICP testing were entered into Microsoft Excel and Quattro Pro spreadsheets for further analysis. Microsoft Excel was used to produce a concise table of results (Table 2) and trend charts for each of the element concentrations and pH of surface water (Figure 4).
Statistical analysis
Quattro Pro spreadsheet software was used for statistical analysis because the t-test function gave more comprehensive results. Three t-tests were run for each element in order to determine if the differences between sample groups were statistically significant. Results from the t-tests can be found in Table 3.
Results and discussion
Samples numbered
1-4 were collected from South Creek along the South Creek Greenway area south
of
Table 3 shows the
levels of significance determined by t-tests on each combination of samples
groups and the associated metal and phosphorus concentrations. It appears that
the SWTP is contributing (or has contributed) significant amounts of
phosphorus, lead and copper to
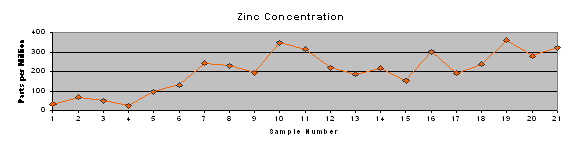
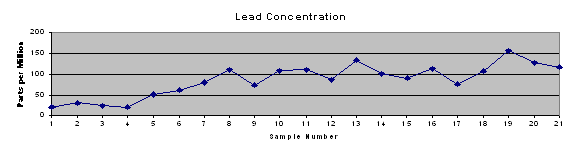
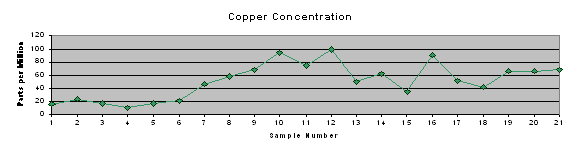
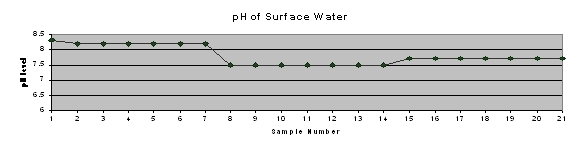
Trend charts were produced (Figure 4) to graphically represent the concentrations of metals and phosphorus at each site, as well as the pH measured in that stretch of stream. It shows the increase in concentrations of each of the metals and phosphorus beginning at or near the SWTP discharge pipe (immediately after sample site 8). Additionally, the metals tend to track each other to some extent. This may indicate a common source, indiscriminate precipitation of all metals on the sediments present in the specific location, or some other process happening in the local “microenvironment.”
Table 2: Concentration of phosphorus and heavy metals in stream
sediments and pH of surface water
|
|
|
|
|
Concentration of Phosphorus and Heavy Metals in Stream Sediments (ppm) |
pH of surface water |
|||
|
|
|
|
|
|||||
|
|
|
|
Sample |
P |
Zn |
Cu |
Pb |
pH |
|
|
|
Upstream South Creek (control) |
1 |
180.5 |
32.1 |
15.5 |
21.6 |
8.3 |
|
|
|
2 |
354.8 |
67.5 |
23.2 |
31.7 |
8.2 |
|
|
|
|
3 |
287.0 |
52.2 |
16.8 |
25.1 |
8.2 |
|
|
|
|
4 |
153.1 |
23.7 |
9.9 |
21.3 |
8.2 |
|
|
|
|
|
Mean value |
243.9 |
43.9 |
16.3 |
24.9 |
8.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Upstream |
5 |
188.7 |
99.4 |
17.2 |
51.9 |
8.2 |
|
|
|
6 |
236.2 |
129.9 |
20.5 |
62.1 |
8.2 |
|
|
|
|
7 |
367.4 |
244.6 |
46.6 |
80.2 |
8.2 |
|
|
|
|
|
Mean Value |
264.1 |
158.0 |
28.1 |
64.7 |
8.2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Downstream |
8 |
1255.4 |
228.6 |
57.7 |
111.1 |
7.5 |
|
|
|
9 |
2150.0 |
197.8 |
68.3 |
74.9 |
7.5 |
|
|
|
|
10 |
3216.0 |
350.6 |
94.4 |
109.5 |
7.5 |
|
|
|
|
11 |
2352.0 |
314.6 |
75.0 |
110.9 |
7.5 |
|
|
|
|
12 |
1426.6 |
221.4 |
99.4 |
86.4 |
7.5 |
|
|
|
|
13 |
721.6 |
187.9 |
49.8 |
135.0 |
7.5 |
|
|
|
|
14 |
1374.8 |
217.4 |
62.0 |
100.6 |
7.5 |
|
|
|
|
15 |
872.0 |
151.6 |
35.4 |
90.4 |
7.7 |
|
|
|
|
16 |
2214.0 |
302.8 |
90.8 |
113.2 |
7.7 |
|
|
|
|
17 |
1573.4 |
192.7 |
51.9 |
77.1 |
7.7 |
|
|
|
|
18 |
997.2 |
238.8 |
41.9 |
106.8 |
7.7 |
|
|
|
|
19 |
686.4 |
362.6 |
66.2 |
157.7 |
7.7 |
|
|
|
|
20 |
354.8 |
279.4 |
65.6 |
128.3 |
7.7 |
|
|
|
|
21 |
1502.0 |
321.6 |
68.1 |
117.3 |
7.7 |
|
|
|
|
|
Mean Value |
1478.3 |
254.8 |
66.2 |
108.5 |
7.6 |
|
|
|
|
|
|
|
|
|
|
Table 3: Confidence of statistical difference between sample groups
|
|
|
|
|
P |
Zn |
Cu |
Pb |
|
Upstream |
p < .001 |
NS |
p < .05 |
p < .05 |
|||
|
Downstream |
|||||||
|
|
|
|
|
|
|
|
|
|
Upstream |
NS |
NS |
NS |
p < .05 |
|||
|
Upstream South Creek (control) |
|||||||
|
|
|
|
|
|
|
|
|
|
Upstream South Creek (control) vs. |
p < .001 |
p < .001 |
p < .001 |
p < .001 |
|||
|
Downstream |
|||||||
|
|
|
|
|
NS = not significant (p>.10) |
|
||
Figure 4: Trend charts of metal and phosphorus concentrations and pH of surface water
South Creek control
![]()
South Creek control
![]()
South Creek control
![]()
South Creek control![]()
South Creek control![]()
Conclusion
The analysis of
the data suggests that concentrations of phosphorus, copper and lead were
emitted from the Southwest Wastewater Treatment Plant into
The Environmental Protection Agency does not currently publish any regulatory standards for toxic metals or nutrients in stream sediments. Without proper studies, it is difficult to state whether or not metals and phosphorus on sediments pose a hazard to aquatic life, how long the precipitates will persist even after concentrations in the effluent are reduced, or whether remediation is necessary. It does seem clear, however, that analysis of sediments in a stream can indicate higher concentrations of pollutants in water, and that the location of the source of the pollution can be indicated by increased concentrations of pollutants in sediments downstream.
Future studies should look at persistence of precipitates in sediments to determine if and when the metals or nutrients might be released. Perhaps as the concentration of metals in the water decreases, the precipitates will dissolve and eventually be diluted to less harmful levels as they are carried in solution downstream. Additionally, studies might determine the affinity of certain metals or nutrients such as phosphorus for different natural sediment types to see if more or less will adsorb to sediments in other geologic settings.
References
Black, J. (1997).
Bullard, L.
(2001). Water Resources of
City of
-----------. (2001). Industrial Pretreatment Program. Retrieved
-----------. (2000). City of
-----------. (2001). Phosphorus – Too Much of a Good Thing. Retrieved
-----------. (2001). Wastewater Collection System – Sanitary Sewer Maintenance
Section. Retrieved
Environmental Working Group. (undated).
Toxic water pollution in
Kiner, L. K. and Vitello, C. (1997?).
Mantei, E. J., Ernst, R. L., and Zhou, Y. (1993). Comparison
of metal homogeneity in grab, quartered, and crushed – sieved portions of
stream sediments and metal content change resulting from crushing – sieving
activity. Environmental Geology, 22, pp. 186-190.
Missouri Department of Natural Resources. (undated). Outreach and
National Park Service. (1998). Water
Pollution. Retrieved
Pulley, T. S., Nimmo, D. R. and Tessari, J. D. (1998). Characterization of toxic conditions above
Thomson, K. C.
(1986). Geology of